Posted on September 3, 2018 by Archer USMLE Reviews
495. A 49-year-old obese female presents with abdominal pain. Patient states the pain acutely onset several hours ago and describes it as constant, severe, localized around the umbilicus and radiating to her back. She says the pain feels better if she is sitting up and bending slightly forward. Patient also says she feels nauseous and vomited once after the pain began. No relevant past medical or surgical history. No current medications or allergies. Review of systems is significant for several recent episodes of abdominal pain after eating.
On exam, the patient is in distress, sitting upright, and leaning slightly forward. Vitals are temperature 38.3 °C (101.1 °F), blood pressure 110/78 mm Hg, pulse 105/min, respirations 25/min, and oxygen saturation 98% on room air. Cardiac exam is normal. Lungs are clear to auscultation. Abdominal exam reveals tenderness to palpation in the periumbilical region. Bowel sounds are diminished.
Laboratory values are:
Sodium 140 mEq/L
Potassium 4.0 mEqL
Chloride 100 mEq/L
Bicarbonate 25 mEq/L
BUN 35 mg/dL
Creatinine 1.1 g mg/dL
Glucose (fasting) 90 mg/dL
Calcium 7.0 mg/dL
Phosphorous 4.1 mg/dL
Bilirubin, conjugated 1.5 mg/dL
Bilirubin, total 3.0 mg/dL
AST (SGOT) 325 mU/mL
ALT (SGPT) 175 mU/mL
Alkaline Phosphatase 295 U/L
Amylase 250 U/L
Lipase 89 U/L
TSH 1.1 mIU/L
PTH 30 pg/mL
Troponin I (cTnI) <0.02 ng/mL
Lactate Deydrogenase (LDH) 750 U/L
C-reactive protein 45 mg/L
b-hCG <1 mIU/mL
WBC 19,000 / mm3
RBC 4 x 106/mm3
Hematocrit 45%
Hemoglobin 13.0 g/dL
Platelet Count 275,000 / mm3
Differential
Neutrophils (%) 85
Lymphocytes (%) 10
Monocytes (%) 5
Eosinophils (%) 3.5
Basophils (%) 1.5
Chest and KUB x-rays, and EKG are unremarkable.
Aggressive fluid resuscitation and supplemental 100% oxygen are given. Meperidine 150 mg intramuscularly is administered. Abdominal ultrasound reveals the presence of a gallstone in the common bile duct (CBD). Patient is kept NPO.
What is the next best step in management?
A. Contrast CT of the abdomen
B. Endoscopic retrograde cholangiopancreatography (ERCP)
C. Administer meropenem 1 g IV every 8 hours
D. Laparoscopic cholecystectomy
E. Magnetic resonance cholangiopancreatography (MRCP)
@hagemanGIstep2
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER GASTROENTEROLOGY, USMLE GASTROENTEROLOGY QUESTIONS, usmle step 3, USMLE STEP 3 GASTROENEROLOGY, usmle step 3 gastroenterology, USMLE Step 3 question bank, usmle step 3 questions | 1 Comment »
Posted on September 2, 2018 by Archer USMLE Reviews
Hurry Up and Register free! Only until January 2019! Total 600 Questions now and counting! Archer Review has partnered with PulsedIn – download PulsedIn social test prep app at https://itunes.apple.com/us/app/pulsedin/id1127822308?mt=8 .
You can then access Archer Review and access Step 1 QBANK for free. Most Qs from USMLE Step 1 Qbank will be also discussed live on PulsedIn app.
Questions added every day – HigHyield and Recall questions. #USMLE #MEDED #FOAMED #MEDSTUDENT
 Archer USMLE Step 1 Blog
Archer USMLE Step 1 Blog
Access Archer HighYield Step 1 Qbank – and practice on exactly similar USMLE exam style Qbank Interface, analyze your strengths, compare with peers, master the concepts at https://archerreview.com/products?subexamid=1
Free 30 day Trial. No Payment information needed to try.
View original post
Filed under: USMLE Test Prep | Leave a comment »
Posted on August 4, 2018 by Archer USMLE Reviews
494. A 45 year old man with a 20-year history of tobacco use presents to the emergency department after having repeated episodes of sharp, stabbing substernal pain with a dry, hacking cough over the last 2-3 days. The episodes last between 5-10 seconds. He first noticed them several days ago.They were not brought on by exertion and they did not improve with rest. He denies fever, shortness of breath, or tachypnea otherwise. He denies any recent sick contacts. The patient’s temperature is 99.2, heart rate is 72, blood pressure is 143/77. Cardiac exam reveals reveals regular rate and rhythm. There are no murmurs, rubs, or gallops. There are scant crackles throughout the lung fields. Laboratory findings are shown below:
WBC (x 10^3 cells/mL): 10.2
Hemoglobin (g/dL): 13.8
Hematocrit (%): 43.4
RBC (x 10^6/mL): 4.9
RDW (%): 13.2
MCV (fL/cell): 97
MCH (pg/cell): 31
MCHC (%): 36
Platelets (x 10^3): 310
Sodium (mEq/L): 141
Potassium (mEq/L): 4.2
Chloride (mEq/L): 105
Bicarbonate (mEq/L): 19
Creatinine (mg/dL): 1.1
Blood Urea Nitrogen (mg/dL): 23
Glucose, fasting (mg/dl): 78
Calcium (g/dL): 9.3
Calcium, ionized (mEq/L): 2.3
What is the best next diagnostic step?
A. Respiratory viral panel
B. EKG
C. Echocardiogram
D. Troponins
E. ANA
@khangstep2
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: USMLE PULMONOLOGY QUESTIONS, USMLE Respiratory Questions, USMLE STEP3 PULMONOLOGY QUESTION | 7 Comments »
Posted on July 29, 2018 by Archer USMLE Reviews
|
 |
| Usmle Step 3 High-yield Concepts & CCS |
| Facebook Group · 128 members |
|
|
| Interact with peers and admin – Learn High-Yield questions and Concepts, Clinical Case Simulations (CCS) strategies, Recall questions of USMLE Step 3 |
|
|
Filed under: USMLE Test Prep | Tagged: ARCHER CCS WORKSHOP, ARCHER STEP3 RAPID REVIEW, usmle step 3 | 1 Comment »
Posted on July 8, 2018 by Archer USMLE Reviews
#493. A 77-year-old male is brought into the Emergency Department after the staff at his nursing home witnessed a syncopal event. Once a week a barber comes to the nursing home to provide haircuts and shaving services. After the barber raised the head of the patient’s bed and started to shave his neck, the patient suddenly lost consciousness. The barber called for help, and the patient was regaining consciousness by the time staff arrived. Within a few minutes the patient was at his baseline mental status and did not lose control of his bladder or bowels or bite his tongue during the episode. The barber didn’t notice any abnormal movements by the patient but reports he was trying a new “natural” shaving foam this morning. The patient has a history of Parkinson’s disease and hypertension. He does not smoke or drink alcohol. Blood pressure is 124/84 mm Hg and heart rate is 92 beats/minute sitting and 128/82 mm Hg and 88 beats/minute after standing two minutes. What would be the next best step in management?
A. Immediate referral for permanent pacemaker
B. Carbamazepine
C. Referral to an Electrophysiologist
D. Carotid endarterectomy
E. Reassurance, education and clinical follow-up
cardio@jamesv
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer cardiology, cardiology questions, USMLE STEP 3 CARDIOLOGY, uworld step 3 | 3 Comments »
Posted on June 27, 2018 by Archer USMLE Reviews
#492. A 37-year-old woman with a history of bulimia nervosa presents with chest pain. Patient describes it as a sharp pain, localized to the middle of the chest, and occurred acutely last night after vomiting. Current medications are fluoxetine 60 mg orally daily and a daily multivitamin.
On physical exam, patient appears ill and diaphoretic. Vital signs show temperature of 37 °C (98.6 °F), blood pressure of 135/85 mm Hg, pulse of 90 /min, respiratory rate of 30 / min, and oxygen saturation of 98% on room air. Oropharynx is erythematous, dentition is poor and back molars show significant erosion. There is dullness to percussion and diminished breath sounds over the lower lobe of the left lung. Remainder of the physical exam is normal. EKG and laboratory tests are normal. Chest x-ray shows a small left pleural effusion and evidence of pneumomediastinum.
Which of the following is the definitive diagnostic step in this patient?
A. Esophagogastroduodenoscopy
B. Barium swallow study
C. Helical CT of the chest without contrast
D. Gastrografin swallow study
E. Thoracentesis
@card-hageman
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer cardiology, USMLE STEP 3 CARDIOLOGY | 4 Comments »
Posted on June 25, 2018 by Archer USMLE Reviews
#491. A 65-year-old African-American male with a long-standing history of coronary artery disease and hypertension presents with shortness of breath and fatigue. Patient says he feels fatigued throughout the day and that he can no longer walk across the room without being short of breath. Patient states that symptoms onset a couple days ago and have steadily worsened.
Vitals are temperature 37 °C (98.6 °F), blood pressure 165/82 mm Hg, pulse 100/min, respirations 18/min, and oxygen saturation 97% on room air. On physical exam, patient appears alert and in no apparent distress. Cardiac exam reveals a load P2 and an S3 gallop. Pulses are regular but have alternating strong and weak beats. Mean jugular venous pressure is 12 cm H20. Pulmonary exam reveals crackles in the lower lung fields bilaterally. Extremities are cool to the touch and there is 2+ pitting edema bilaterally.
Laboratory tests show:
Sodium 120 mEq/L
Potassium 4.0 mEqL
Chloride 100 mEq/L
Bicarbonate 25 mEq/L
BUN 45 mg/dL
Creatinine 1.0 g mg/dL
Glucose (fasting) 90 mg/dL
Magnesium 1.7 mg/dL
Calcium 9.0 mg/dL
Phosphorous 4.1 mg/dL
Bilirubin, conjugated 0.2 mg/dL
Bilirubin, total 1.0 mg/dL
AST (SGOT) 20 mU/mL
ALT (SGPT) 15 mU/mL
Alkaline Phosphatase 80 U/L
WBC 7000 / mm3
RBC 5 x 106/mm3
Hematocrit 45%
Hemoglobin 15.0 g/dL
Platelet Count 250,000 / mm3
BNP 750 pg/mL
NT-proBNP 1250 pg/mL
Troponin T (cTnT) 0.05 ng/mL
Troponin I (cTnI) 0.03 ng/mL
Chest X-ray reveals evidence of pulmonary vascular congestion and pulmonary edema in the lung bases bilaterally. EKG reveals Q wavs in leads V1 and V2, evidence of left ventricular hypertrophy and a left bundle branch block. Transthoracic echocardiography reveals a LV ejection fraction of 25%, increased left ventricular wall thickness, moderate left atrial enlargement, and evidence of left anterior wall dyskinesis consistent with an old anterior wall myocardial infarction.
Patient is admitted to CCU and started on high flow 100% oxygen by nasal canula. Carvedilol 25 mg orally twice daily, captopril 25 mg orally three times daily, furosemide 100 mg orally, and eplerenone 25 mg orally daily are initiated.
Which of the following is the next best step in management of this patient
A. Administer 1 L NS bolus and then NS + 20 mEq/L KCL IV at 115 mL/h
B. Initiate hydralazine 35 mg orally 3 times daily and isosorbide dinitrate 20 mg orally 3 times daily.
C. Restrict sodium intake to 5 g/day
D. Initiate digoxin 4 mcg/kg orally daily
E. Initiate diltiazem 150 mg orally daily
@card-hageman
Filed under: USMLE Test Prep | 4 Comments »
Posted on February 10, 2018 by Archer USMLE Reviews
A 65-year-old male patient is brought to the emergency department by his relatives for sudden onset of shortness of breath. He was recently diagnosed with bronchogenic carcinoma of the small cell variety. He had come in with a history of coughing up blood on several occasions with a persistent cough. He had been smoking two packs of cigarettes a day for the last 20 years. Today he also complains of occasional headaches and a difficulty in seeing. He complains that these symptoms are more prominent in the morning when he wakes up from sleep. It subsides as the day progresses. Which of the following are the further physical findings the physician is expected to find in this patient?
A. Lower extremity edema along with distention of the superficial veins of the abdomen.
B. Edema and a purplish hue in the upper extremities, extending to the head.
C. Low blood pressure and a weak pulse, with extended jugular veins.
D. Breathing produces paradoxical movements in the chest.
E. Swelling of the feet with an increased shortness of breath as the patient lies down.
Filed under: USMLE STEP 1 Qbank, USMLE Test Prep | Tagged: archerusmle, pathology, usmle pathology questions, usmle step 1, usmle1, usmlestep1 | 3 Comments »
Posted on February 10, 2018 by Archer USMLE Reviews
Saturday challenge! Answer this question. Here is how a typical Archer Step 1 Qbank interface looks, simulates USMLE Step 1 exam exactly. Try more at
USMLE MICROBIOLOGY
Filed under: USMLE STEP 1 Qbank, USMLE Test Prep | Tagged: archerusmle, microbiology question, usmle microbiology, usmle step 1, usmle1, usmlestep1 | 1 Comment »
Posted on February 10, 2018 by Archer USMLE Reviews
Archer launches USMLE Step 1 Qbank with detailed explanations and analysis. Test and stimulate your brain with intriguing high-yield Step 1 concepts that have been repeatedly tested on USMLE Step 1. Archer has been a leader in Step 3 preparation courses for over 10 years and enjoys huge base of satisfied customers. We have decided to apply same level of diligence and foundations to our Step 1 Q-bank.
Another goal of Archer is to make Q-banks very affordable to students who are already struggling with student loans, credit card debts and other expenses. Average cost for qbank from UWORLD and Kaplan for just 1 month subscription is about $220. This is a huge burden for struggling students. Archer will give you most updated, highest quality, brand new question banks with all analytical tools and features exactly similar to your original test interface at extremely low price of $49 for 30 days. Archer Step 1 Qbank has 500 Qs and Step 3 qbank has 800 questions. New questions being added almost daily.
Best quality at extremely affordable prices. We have been there before in your shoes and we understand the financial constraints. By helping you with lowest priced highest quality q-banks, we aim to grow honestly .
Join the revolution! @ https://ArcherReview.com . Follow us on twitter @USMLEGALAXY
Filed under: USMLE Test Prep | Tagged: archerusmle, usmle step 1, USMLE STEP1, usmle1, usmlestep1 | 1 Comment »
Posted on January 21, 2018 by Archer USMLE Reviews
Live Workshop/ Webinar date: JANUARY 27TH 2018
Interactive practice of USMLE Step3 CCS Cases.
The most time efficient strategy to pass USMLE Step 3! A component of Archer Live USMLE Reviews. Schedule ( Starts at 10 AM CDT i.e; 11 AM EST)
10 AM to 10:15 AM – Intro on the Webinar functions
10:15 AM to 12:15 PM – Recognizing Unstable vitals. – General approach to ER cases– Real time vs. Simulated time – Strategies to keep Simulated time low (ER) – Demonstration of high scoring strategies
12:15 pm to 12:30 pm – Q and A session
12:30 PM to 1:30 PM – Office case manAgement principles – General approach to office cases – Indications for admisiion – Scheduling follow up tests – Navigating the clock with ease to score more. – Demonstration of office cases
1:30 PM to 2:30 PM – Q and A session
2:30 PM to 4:00 PM – Lunch break
4:00 PM to 5:30 PM – Practice of very High Yield cases with one on one Q and A sessions
5:30 PM to 5:45 PM – Break
5:45 PM to 7:45 PM – Practice of Very Highyield CCS cases
7:45 PM to 8:00 PM – Break
8:00 PM to 9:00 PM – Case Practice, discussion and wrap-up!
Can’t make it to the Webinar ? Have exam before next live session? : PPV recordings of most high-yield CCS workshops are readily available at https://archerreview.com/products?subexamid=4 Price: $88 and you may access those 24/7.
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER CCS WORKSHOP, ARCHER STEP3 LIVE REVIEWS, ARCHER STEP3 RAPID REVIEW, USMLE step 3 CCS, usmle step 3 live review, USMLE STEP3 CCS WORKSHOP, UWORLD CCS | Leave a comment »
Posted on January 14, 2018 by Archer USMLE Reviews
Posted on January 14, 2018 by Archer USMLE Reviews
 Archer USMLE Step 3 Blog
Archer USMLE Step 3 Blog
Approaching Prolonged PTT and understanding Mixing Studies :
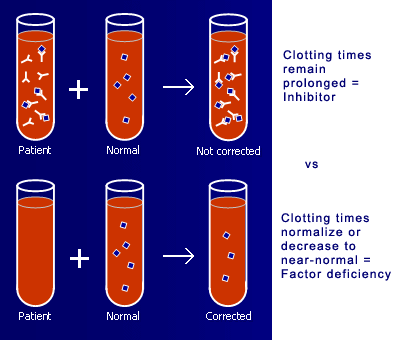 ARCHER HEMATOLOGY REVIEW
ARCHER HEMATOLOGY REVIEW
Question : On a mixing study, the PTT corrects initially but gets prolonged again after incubation for 2 hours. What is this condition?
This description of mixing studies is consistent with presence of a temperature- and time-dependent anti-VIII inhibitor. It just means that the inhibitor is a warm reacting IgG antibody that requires one or two hour’s incubation at 37°C to be detected. If the PTT corrects initially but prolongs again after incubation (meaning if thePTT is at least 15% longer than the normal reagent plasma’s incubated PTT ) –> anti-VIII should be suspected. This can be confirmed by obtaining a Factor VIII level which will be low in case of presence of inhibitor. Further coinfirmation of Factor VIII inhibitor can be obtained by Bethesda Titer. In such cases, you should also carefully review patient’s prior bleeding history –…
View original post 463 more words
Filed under: USMLE Test Prep | Leave a comment »
Posted on August 9, 2017 by Archer USMLE Reviews
490. An 18-year-old male presents to the Emergency Department for evaluation of chest pressure for an hour. The pain is radiating to his jaw. On examination, he is diaphoretic, and he complains of nausea. Past medical history is unremarkable. Urine reveals positive benzolegonine. Below is his ECG. What is the most likely cause of the patient’s symptoms?

A. Early repolarization
B. Ischemia
C. Cocaine induced myocardial infarction
D. Atrial fibrillation
E. Wolf-Parkinson-White Syndrome
Filed under: USMLE Step 2CK Qbank, USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer cardiology, ARCHER ELECTROCARDIOGRAMS, ARCHER STEP3 LIVE REVIEWS, ARCHER STEP3 RAPID REVIEW, USMLE STEP 3 CARDIOLOGY, USMLE STEP 3 ELECTROCARDIOGRAMS, USMLE Step3, USMLE STEP3 PREPERATION | 5 Comments »
Posted on August 5, 2017 by Archer USMLE Reviews
A 35 Year old woman presents for evaluation of recurrent headaches for the past few months. Lately, she has been waking up with a headache almost daily though it tends to improve in the latter half of the day. She is frustrated as she can not even get some sleep during the attack since lying down makes her feel worse . She has associated nausea. She has transient attacks of light flashes that resolve spontaneously. Her menstrual history is unremarkable. On physical examination, she has no neurological deficits. Funduscopic examination shown below: 
Which of the following information, if elicited in the patient history, would increase the clinical suspicion of the diagnosis?
A) Constipation
B) Family history
C) Recent weight gain
D) Galactorrhea
E) Urinary Incontinence
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer neurology, neurology mcq, usmle step 3 neurology, usmle step 3 review course | 3 Comments »
Posted on August 5, 2017 by Archer USMLE Reviews
A 31 Year old woman diagnosed with suspected diagnosis of Idiopathic intracranial hypertension underwent a lumbar puncture for diagnostic purposes. An MRI of the brain that was obtained prior to lumbar puncture did not reveal any structural abnormalities or mass effect. Laboratory investigations including coagulation parameters were normal. About 12 hours after the procedure, the patient complains of moderate to severe headache. Headache is mainly in the occipital region which increases while sitting up and improves on lying flat. She has two episodes of vomiting in the last one hour. She also complains of dizziness and ringing sensation in her ears. Physical examination does not reveal any papilledema or focal neurological deficits.
Most appropriate next step in managing this patient?
A) Blood cultures
B) MRI of the Lumbar Spine
C) Non-Contrast CT scan of brain
D) Observation
E) Acetazolamide
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer neurology, usmle step 3 courses, usmle step 3 neurology, usmle step 3 review, usmle step 3 review course | 3 Comments »
Posted on June 11, 2017 by Archer USMLE Reviews
487) 57-year-old male with a history of mild COPD has been using albuterol as needed to manage his COPD without any other maintenance medications. Recently he has been experiencing a greater degree of shortness of breath, wheezing and a productive cough. Pulmonary function tests demonstrate a FEV1 that is 60% of predicted. What is the next best step for management of his COPD?
A. Add budesonide to treatment regimen
B. Add rofilumast to treatment regimen
C. Add tiotropium to treatment regimen
D. Add tiotropium plus fluticasone to treatment regimen
E. Add carbocystine to treatment regimen
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer pulmonology, archer usmle step 3, COPD MANAGEMENT, GOLD COPD GUIDELINES, usmle step 3, USMLE STEP 3 PULMONOLOGY | 6 Comments »
Posted on June 11, 2017 by Archer USMLE Reviews
A 56-year-old male with Type II diabetes presents to you today for a follow-up visit. Three months prior his A1c was 8.9% and he was started on metformin. Today his A1c is 7.4%. He is significantly overweight with a BMI of 33. You would like to initiate another medication for glucose control, specifically one that does not carry a risk of weight gain. Which of the following drugs is weight neutral?
- A. Glimepride
- B. Rosiglitazone
- C. Pioglitazone
- D. Sitagliptin
- E. Glyburide
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer endocrinology, ARCHER STEP3 LIVE REVIEWS, usmle step 3, usmle step 3 endocrinology | 5 Comments »
Posted on January 7, 2017 by Archer USMLE Reviews
A 48-year-old homeless man presenting with severe abdominal pain. He has been hospitalized 6 times in the past year for similar complaints. Hospital records indicate multiple admissions with alcohol intoxication. The pain radiates to his back, and is accompanied by nausea and vomiting. The patient has a temperature of 103F, but does not permit an abdominal exam. Laboratory analysis indicates elevated gamma glutamyl transpeptidase and an AST/ALT ratio > 2. What is the most likely diagnosis?
A – Cholecystitis
B – Pancreatitis
C – Choledocholithiasis
D – Duodenal peptic ulcer
E – Gallbladder cancer
Filed under: USMLE Step 2CK Qbank, USMLE Test Prep | Tagged: ARCHER GASTROENTEROLOGY, usmle step 2 ck, USMLE STEP2 GASTROENTEROLOGY, USMLE STEP2CK QBANK | 7 Comments »
Posted on January 7, 2017 by Archer USMLE Reviews
485. A 10-year-old female presents with a 3 week history of cough. Initially she had a runny nose and was tired with a slight cough but as the runny nose resolved the cough seemed to get worse. She states that the cough is dry sounding and occurs during the day and night. She describes having coughing spasms that occasionally end in vomiting but between episodes of coughing she is fine. Her mom reports that during a coughing spasm she will gasp for air and sometimes make a “whooping” noise. A nasopharyngeal swab confirms a diagnosis of Bordatella pertussis. Which of the following is true?
A. Her 3-month-old brother should be treated with azithromycin as prophylaxis
B. She should be started on azithromycin for more rapid resolution of cough.
C. Her classmates should be treated with clarithromycin as prophylaxis
D. She will have lifelong natural immunity against Bordatella pertussis.
E. Her classmates should receive a Tdap booster regardless of their vaccination status
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Infectious Diseases, archer pediatrics, ARCHER STEP3 LIVE REVIEWS, usmle step 3, USMLE Step3 | 11 Comments »
Posted on January 1, 2017 by Archer USMLE Reviews
A 50-year-old African american woman presents to your clinic with increased thirst and increased urination. You review her old records and it appears like she had a Chest X-ray in the ER last year that showed bilateral hilar lymphadenopathy. She was advised follow-up but she did not comply at that time. Upon examination, you discover some subcutaneous skin nodules and biopsy of one of these comes back positive for non-caseating granulomas.
Laboratory studies from a week ago show:
Hemoglobin 15.2gm%
Calcium : 11.6mg%
Creatinine : 1.0mg%
Which of the following investigations may explain the underlying mechanism of her Hypercalcemia?
A. Biopsy of Hilar Lymph Node
B. 25 Hydroxy Vitamin D Level
C. PTH related peptides
D. 1,25 di-hydroxy Vitamin D level
E. Serum Protein Electrophoresis
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER FLUID AND ELECTROLYTES, ARCHER NEPHROLOGY, ARCHER STEP3 LIVE REVIEWS, ARCHER STEP3 RAPID REVIEW, Foundations of Independent Practice (FIP), lab interpretation, Pathophysiology correlation, usmle step 3 nephrology | 8 Comments »
Posted on January 1, 2017 by Archer USMLE Reviews
A 50-year-old previously healthy man comes to his physician for a scheduled follow-up examination. He works as an electrician, which involves a significant amount of physical activity when working. He says he also runs 5 miles twice a week and is on a low-fat, low-cholesterol diet. His father died of a ‘heart attack’ at the age of 54, and he has smoked one pack of cigarettes daily for the past 25 years. He denies alcohol or illicit drug use. He says he leads a low-stress lifestyle and has no complaints. His current medication include lisinopril. On presentation, his blood pressure is 135/85 mm Hg. Laboratory studies from a week ago show:
Total cholesterol 287 mg/dL
HDL 65 mg/dL
LDL 180 mg/dL
Which of the following is the best next step in patient care?
| A |
Initiate drug therapy for control of his hyperlipidemia |
| B |
Educate the patient about diet and exercise and repeat the tests within 4 weeks |
| C |
Educate the patient about diet and exercise and repeat the tests within a year |
| D |
Initiate a mandatory low-fat diet for the patient |
| E |
No intervention is indicated |
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: approach to hyperlipidemia, archer preventive medicine, choosing hyperlipidemia therapy, Goals in treating hyperlipidemia, usmle step 3 preventive medicine | 6 Comments »
Posted on January 1, 2017 by Archer USMLE Reviews
A 63-year-old woman comes to the emergency department complaining of severe midabdominal pain. She reports that the pain has increased in intensity over the past few days. There has been no associated nausea or vomiting, no change in bowel habits, and no relief afforded by position changes. She is postmenopausal and does not take hormone replacement therapy. She has a 30-year history of hypertension, and has been noncompliant with her therapy of calcium channel blocker and thiazide diuretic. On examination her abdomen is obese but there is a suggestion of a nontender, pulsatile mass in the epigastric region. The remainder of the physical examination is normal. Which of the following is the best next step in management?
| A. Abdominal ultrasound |
| B. Abdominal CT scan without contrast |
| C. Abdominal CT scan with contrast |
| D. Angiography |
| E. Observation |
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER GASTROENTEROLOGY, archer surgery, usmle step 3 surgery | 11 Comments »
Posted on November 24, 2016 by Archer USMLE Reviews
BACKGROUND:
Beta-blockers reduce mortality in patients who have chronic heart failure, systolic dysfunction, and are on background treatment with diuretics and angiotensin-converting enzyme inhibitors. We aimed to compare the effects of carvedilol and metoprolol on clinical outcome.
METHODS:
In a multicenter, double blind, and randomized parallel group trial, we assigned 1511 patients with chronic heart failure to treatment with carvedilol (target dose 25 mg twice daily) and 1518 to metoprolol (metoprolol tartrate, target dose 50 mg twice daily). Patients were required to have chronic heart failure (NYHA II-IV), previous admission for a cardiovascular reason, an ejection fraction of less than 0.35, and to have been treated optimally with diuretics and angiotensin-converting enzyme inhibitors unless not tolerated. The primary endpoints were all-cause mortality and the composite endpoint of all-cause mortality or all-cause admission. Analysis was done by intention to treat.
FINDINGS:
The mean study duration was 58 months (SD 6). The mean ejection fraction was 0.26 (0.07) and the mean age 62 years (11). The all-cause mortality was 34% (512 of 1511) for carvedilol and 40% (600 of 1518) for metoprolol (hazard ratio 0.83 [95% CI 0.74-0.93], p=0.0017). The reduction of all-cause mortality was consistent across predefined subgroups. Incidence of side effects and drug withdrawals did not differ by much between the two study groups.
- To which of the following patients are the results of this clinical trial applicable?
- A 62-year-old male with primarily diastolic congestive heart failure.
- A 75-year-old female with systolic dysfunction and an EF of 45%.
- A 56-year-old male with NYHA class I systolic heart failure.
- A newly diagnosed 66-year-old male who has yet to begin treatment for his NYHA class II systolic heart failure.
- A 68-year-old male with NYHA class II systolic heart failure and EF 30%.
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer USMLE RESEARCH ABSTRACT QUESTIONS, Research Abstract Questions, Research Abstract Questions Step 3, usmle step 3 | 4 Comments »
Posted on October 29, 2016 by Archer USMLE Reviews
RESEARCH ABSTRACT QUESTION #1
BACKGROUND:
Aldosterone blockade reduces mortality and morbidity among patients with severe heart failure. We conducted a double blind, placebo-controlled study evaluating the effect of eplerenone, a selective aldosterone blocker, on morbidity and mortality among patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure.
METHODS:
Patients were randomly assigned to eplerenone (25 mg per day initially, titrated to a maximum of 50 mg per day; 3319 patients) or placebo (3313 patients) in addition to optimal medical therapy. The study continued until 1012 deaths occurred. The primary end points were death from any cause and death from cardiovascular causes or hospitalization for heart failure, acute myocardial infarction, stroke, or ventricular arrhythmia.
RESULTS:
During a mean follow-up of 16 months, there were 478 deaths in the eplerenone group and 554 deaths in the placebo group (relative risk, 0.85; 95 percent confidence interval, 0.75 to 0.96; P=0.008). Of these deaths, 407 in the eplerenone group and 483 in the placebo group were attributed to cardiovascular causes (relative risk, 0.83; 95 percent confidence interval, 0.72 to 0.94; P=0.005). The rate of the other primary end point, death from cardiovascular causes or hospitalization for cardiovascular events, was reduced by eplerenone (relative risk, 0.87; 95 percent confidence interval, 0.79 to 0.95; P=0.002), as was the secondary end point of death from any cause or any hospitalization (relative risk, 0.92; 95 percent confidence interval, 0.86 to 0.98; P=0.02). There was also a reduction in the rate of sudden death from cardiac causes (relative risk, 0.79; 95 percent confidence interval, 0.64 to 0.97; P=0.03). The rate of serious hyperkalemia was 5.5 percent in the eplerenone group and 3.9 percent in the placebo group (P=0.002), whereas the rate of hypokalemia was 8.4 percent in the eplerenone group and 13.1 percent in the placebo group (P<0.001).
- Which of the following statements represents the most accurate interpretation of the results from the aforementioned clinical trial?
- There was no significant difference in the incidence of hyperkalemia between trial arms.
- There was no significant difference in the rate of sudden cardiac death between trial arms.
- Epleranone, when added to optimal medical therapy, decreases all cause mortality in patients with left ventricular dysfunction following myocardial infarction.
- The rate of hypokalemia was not significantly different between trial arms.
- The most common causes of death seen in enrolled patients over the course of this trial were non-cardiac in nature.
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Bio-statistics, Archer USMLE RESEARCH ABSTRACT QUESTIONS, Research Abstract Questions, Research Abstract Questions Step 3, USMLE Step 3 Bio-statistics | 4 Comments »
Posted on September 18, 2016 by Archer USMLE Reviews
A 90 year old man with history of advanced dementia is brought to hospital with aspiration pneumonia. This is his 5th episode of Aspiration pneumonia in the past 1 year. He is accompanied by his daughter who also happens to be his healthcare proxy. She wants to focus on her father’s comfort as the goal of care and requests no further diagnostic interventions and she would like to avoid further hospitalizations. She wants to know what would make him eligible to receive hospice care. Based on which of the following factors, can he be qualified for hospice?
A. His advanced age
B. Diagnosis of advanced dementia
C. Prognosis
D. His wishes to die at home
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER ETHICS, Hospice, Hospice Care, Hospice Eligibility, Hospice qualify, usmle step 3 ethics | 16 Comments »
Posted on March 27, 2016 by Archer USMLE Reviews
News from USMLE :
USMLE Step 3 Change in number of items and score delay
Beginning the week of January 18, 2016, the number of items on the Step 3 examination will decrease. There will be a delay in reporting scores for exams administered between January 18 and April 30, 2016. The target date for reporting Step 3 scores for most examinees testing during this time period is May 25, 2016.
Although this change will occur quickly at many test centers, there may be some locations where the transition takes longer to complete. The overall transition period should last approximately six weeks. Please note that:
- The length of the testing days will not change.
- Day 1 (Foundations of Independent Practice [FIP]) will continue to be an approximately 7-hour testing session, including time for breaks and tutorials.
- Day 2 (Advanced Clinical Medicine [ACM]) will continue to be a 9-hour testing session, including time for breaks and tutorials.
- Day 1 (FIP) will continue to be divided into six 60-minute blocks.
- Each FIP block will have 38 to 40 multiple-choice questions (MCQs).
- The total number of MCQs on the FIP portion of the examination will be 233.
- Day 2 (ACM) will continue to be divided into six 45-minute blocks of MCQs, and 13 computer-based case simulations (CCS).
- Each ACM MCQ block will have 30 items.
- The total number of MCQ items on the ACM portion of the examination will be 180.
- Scores on examination forms taken before and after the change – as well as scores on forms with different numbers of items – will be comparable; the possible variation in the number of items per form will be accounted for in scoring the examination.
Score reports for Step 3 are usually available within four weeks of testing. However, because of the change described above, as well as routine modifications to the test item pool, score reporting for most Step 3 examinations administered from January 18, 2016 through April 30, 2016 will take longer. As noted above, the target date for reporting Step 3 scores for most examinees testing during this time period is May 25, 2016.
Read the original post at http://www.usmle.org/announcements/default.aspx
#USMLE STEP3
Filed under: USMLE Test Prep | Tagged: usmle step 3 questions | Leave a comment »
Posted on March 2, 2016 by Archer USMLE Reviews
A 32-year-old man comes in regularly because his back hurts. You suspect malingering because he continually requests narcotic pain relievers and imaging studies of his spine. He has come 5 times in the last 6 months with the same problem, typically stating that the pain improves with exercise and leaning forward but is worse at night; pain also occurs at times in his buttocks and does not improve with rest. On physical examination you find no abnormalities of the spine besides inflexibility of the lower spine when he leans forward. Neurological examination is normal, including the absence of pain on a straight-leg raise test. X-ray of the spine and sacroiliac joint is normal. All blood tests including rheumatoid factor, ESR, and C-reactive protein are normal. Today, he again claims to have only minimal improvement with ibuprofen. He is requesting narcotics and a “doctor’s note to get out of work.” What is the next best step in management?
| A. MRI of the sacroiliac joint |
| B. Anti-cyclic citrullinated peptide (anti-CCP) |
| C. HLA-B27 testing |
| D. Methotrexate |
| E. Report to employer for malingering |
|
Filed under: Uncategorized, USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Rheumatology, USMLE step 3 Rheumatology | 29 Comments »
Posted on November 15, 2015 by Archer USMLE Reviews
479. A 57 year old female presents for second opinion of a left breast lesion that had been diagnosed as ‘eczema’ by her regular nurse practitioner, which briefly improved after a short course of topical steroids. On physical exam there is an erythematous, scaly lesion involving the left breast. Image is shown below. Her last mammogram was a year ago and it was “fine”. She does not report any family history of breast cancer. What is the next step in the patient’s management?

A – Bilateral breast ultrasound
B – Bilateral mammography and punch biopsy of nipple
C – Maintain regular annual mammography appointment
D – Oral corticosteroids
E – Left breast MRI
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer dermatology, archer general internal medicine, Archer Gynecology, internal medicine board review, USMLE STEP 3 DERMATOLOGY, usmle step 3 gynecology | 18 Comments »
Posted on November 15, 2015 by Archer USMLE Reviews
478) You are evaluating a 24-year-old female patient who presented with severe abdominal pain two hours prior to admission. She states the pain began in the periumbilical region and has moved to the low abdomen, and she had one episode of emesis with nausea, but no diarrhea. Her last menstrual period was normal and began 27 days ago. On examination, she has a temperature of 98.9, heart rate of 118 beats per minute, and respirations 20/min. Her blood pressure is 100/80. Examination of the abdomen reveals diffuse tenderness to palpation, and she is guarding moderately and has diminished bowel sounds. Complete blood count reveals a hemoglobin of 12 g/dL and a hematocrit of 38%. White blood cell count is 12,500/mm3 with a left shift. Urinalysis reveals 2-5 WBC/hpf. Which of the following is the next best step in management of this patient?
A. Flat and upright abdominal film
B. Pelvic ultrasound
C. Urine b HCG
D. CT abdomen and pelvis
E. Serum beta HCG
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer obstetrics, usmle step 3 gynecology and obstetrics, usmle step 3 obstetrics | 15 Comments »
Posted on September 16, 2015 by Archer USMLE Reviews
| 477) A 39-year-old woman presents to clinic with a history of long-standing right leg pain. She complains of “achy” pain at the hip radiating down her femur to the knee, which is exacerbated on exertion, especially with jogging more so than climbing stairs, but also present during rest. She takes acetaminophen for it, which helps her pain. Her past medical history is significant for systemic lupus erythematosus diagnosed at age of 20, for which she has been taking on and off oral prednisone. She does not smoke cigarettes but he drinks alcohol 2 or 3 times per week. She denies any drug use. Physical examination is significant for limited range of motion of the right hip, especially with external rotation. A plain x-ray shows a dulling of the femoral head but no other pathology. What is the next step in diagnosis? |
| A. Bone scan |
|
| B. CT scan |
|
| C. MRI scan |
|
| D. PET scan |
|
| E. Ultrasound |
|
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: aRCHER ORTHOPEDICS, USMLE STEP 3 ORTHOPEDICS | 4 Comments »
Posted on September 14, 2015 by Archer USMLE Reviews
476. You are evaluating a 12 month old male patient for rhinorrhea and poor appetite. He is friendly and alert. His temperature today is 103.6 degrees Fahrenheit, and his examination is significant for clear nasal discharge and multiple small vesicles on the anterior tonsillar pillars and posterior palate. His examination is otherwise normal. He is accompanied by his mother who seems to be very concerned. The patient has been feeding well and all his developmental milestones have been timely so far. A complete blood count is normal. Which of the following is his most likely diagnosis?

12 month old male patient for rhinorrhea and poor appetite, temperature 103.6 F.
A. Hand, foot, and mouth disease
B. Apthous stomatitis
C. Herpangina
D. Stevens-Johnson syndrome
E. Kawasaki disease
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer pediatrics, USMLE STEP3 PEDIATRICS | 5 Comments »
Posted on September 14, 2015 by Archer USMLE Reviews
A 2-week-old infant is brought to the office for a regular postnatal follow-up. She was born after an uneventful term pregnancy to a 29-year-old G2P2 woman. Mother reports that the infant is feeding well and seems active. No evidence of any fever. The parents are worried about the ‘rash’ that appeared over the infant’s left cheek. On examination, a lesion is noted on the infant’s left cheek as shown below. Vital signs are within normal limits. What is the most appropriate next step in management of this finding?

| A |
Surgical excision of the entire lesion |
| B |
Biopsy of the lesion |
| C |
Laser removal of the lesion |
| D |
Reassurance and observation |
| E |
Topical 5-fluoro-uracil |
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer dermatology, archer pediatrics, dermatology usmle, dermatology usmle step 3 questions, USMLE STEP 3 DERMATOLOGY, usmle step 3 dermatology images, usmle step 3 pediatrics | 9 Comments »
Posted on September 13, 2015 by Archer USMLE Reviews
474. A cohort study is done concerning the relationship between exposure to oral contraception and the risk of breast cancer. 1000 women were followed from 2003 to 2007. Results are listed below:
|
Breast Cancer + |
Breast Cancer – |
|
| Oral contraceptive + |
300 (a) |
200 (b) |
a + b = 500
Probability of breast cancer among exposed: 300/500 = 0.6 |
| Oral Contraceptive – |
100 © |
400 (d) |
c + d = 500
Probability of breast cancer among those not exposed = 100/500 = 0.2 |
|
a + c = 400 |
b + d = 600 |
|
Which of the following represents the absolute risk reduction of breast cancer in women who do not use oral contraceptives?
- 75%
- 67
- 40%
- 50%
- 30%
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Bio-statistics, USMLE Step 3 Bio-statistics | 2 Comments »
Posted on September 13, 2015 by Archer USMLE Reviews
- You are reading a study that was conducted to evaluate IQ (intelligence quotient) scores for patients with bipolar disease. Ten patients did not complete the test. Two patients refused to begin the test. Results for the 94 patients who completed the test revealed an average IQ of 130 with a standard deviation of 20. Which of the following best represents the 95% confidence interval for this study?
- 4
- 2
- 1
- .05
- .1
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Bio-statistics, USMLE Step 3 Bio-statistics | 5 Comments »
Posted on September 10, 2015 by Archer USMLE Reviews
| A 9-year-old boy is brought to the hospital with a wound to his left hand. He was in a fight with peer who bit him. His past medical history is unremarkable and he takes no medications. He has no known allergies. On examination, the child is crying. There is a 2-cm wound over the dorsum of his palm. What is the most appropriate course of action? |
| A. Clean the wound and prescribe amoxicillin-clavulanate. |
|
| B. Clean the wound and prescribe doxycycline. |
|
| C. Clean the wound and prescribe ciprofloxacin. |
|
| D. Clean the wound, close it with interrupted sutures and prescribe ciprofloxacin. |
|
| E. Clean the wound, close it with interrupted sutures and prescribe amoxicillin-clavulanate. |
|
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Infectious Diseases, archer pediatrics, USMLE STEP 3 INFECTIOUS DISEASES, USMLE STEP3 PEDIATRICS | 5 Comments »
Posted on September 10, 2015 by Archer USMLE Reviews
A 14-year-old girl with a history of Crohn’s disease presents with 2-day history of feeling ‘feverish’, malaise, lower abdominal pain and non-bloody diarrhea. She has about 10 episodes of diarrhea daily. She has had three similar episodes in the past 7 years. She denies any recent travel or sick contacts, rush, cough or other symptoms of infection. She has no known allergies and does not take any medications currently. She has been taking 5-ASA, but stopped 6 months ago as she had been asymptomatic for 10 months before that. On presentation, the patient is afebrile with a body temperature of 101.0 F and appears ill. Her blood pressure is 120/80 mmHg and heart rate of 90 beats/minute. Abdominal examination is remarkable for abdominal tenderness in the lower abdomen, but no rebound or guarding. A subsequent CT scan shows dilatation of the left colonic lumen, as well as a thickened colon wall with pericolic fat stranding, particularly in the left colon. The admitting team determines the patient’s presentation is due to exacerbation of her disease. The patient is started on 5-ASA, metronidazole and prednisone and her condition improves within 10 days when she is symptom-free. What is the most appropriate plan after her symptoms have resolved?
| A) Continue 5-ASA, taper prednisone and discontinue metronidazole |
| B) Continue 5-ASA and prednisone, discontinue metronidazole |
| C) Discontinue ASA-5 and metronidazole and continue prednisone for 4 weeks, followed by taper |
| D) Recommend therapy with infliximab an instruct to take 5-ASA when she starts experiencing symptoms, discontinue prednisone and metronidazole |
| E) Continue 5-ASA and metronidazole for at least 4 weeks, taper prednisone |
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER GASTROENTEROLOGY, archer pediatrics, USMLE STEP 3 GASTROENEROLOGY, USMLE STEP3 PEDIATRICS | 4 Comments »
Posted on April 14, 2015 by Archer USMLE Reviews
470) An 11-year-old with a history of asthma and seasonal allergies is currently using albuterol to manage asthma symptoms. Recently his use of albuterol has increased from 1-2 days per week to 4 times per week for several weeks, though does not experience his symptoms daily. On physical examination, you find that his vital signs are within normal limits. Auscultation of his lungs reveals mild end expiratory wheezing. Given his persistent symptoms, what change should be made to his current treatment regimen?
A. Add salmeterol twice daily
B. Add montelukast 10mg daily
C. Add fluticasone daily
D. Add formoterol + budesonide twice daily
E. Add tiotropium
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer pulmonology, archer step 3 pulmonology, USMLE STEP 3 PULMONOLOGY | 11 Comments »
Posted on April 6, 2015 by Archer USMLE Reviews
| A study is performed to evaluate the incidence of peri-operative stroke during a carotid endarterectomy based on whether the surgeon uses a shunt during the procedure or not. The authors calculate the odds ratio of suffering from stroke if the surgeon uses a shunt is 0.84 (95% CI: 0.72 – 1.12) compared to when the surgeon does not use a shunt. What is the most appropriate interpretation of this finding? |
| A. Use of shunt decreases risk of stroke by 84% |
|
| B. Use of a shunt does not decrease or increase risk of stroke |
|
| C. Use of shunt does not decreases the risk of stroke |
|
| D. Use of shunt decreases risk of stroke with a 0.84 odds ratio |
|
| E. Use of shunt decreases risk of stroke by 16% |
|
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Bio-statistics, USMLE Step 3 Bio-statistics | 14 Comments »
Posted on March 26, 2015 by Archer USMLE Reviews
Congratulations to everyone who passed! Results were held over the past 5 months and were just released. We just received over-whelming number of e-mails from our students reporting their scores and pass/ fail status. About 95% of our students have passed and that is very encouraging. This should allay the fears about new Step 3. New format Step 3 has increased representation of bio-statistics, epidemiology, ethics and preventive medicine/ screening guidelines. Number of students were actually quite worried about increased proportion of Bio-statistics questions but it appears like most of them have done really well on that part of the exam. Looking at some of the score reports even those who failed the exam have surprisingly done well on bio-statistics and other multiple choice questions. Failures predominantly happened again because of not placing enough emphasis on CCS preparation and scoring too low on the CCS. Improving on CCS and moving your performance graphs towards the right ( good to excellent performance) may help you pass much easily even when you do just average or slightly below-average on the MCQs. Scoring higher on CCS reduces your burden by easing off the requirement to answer more number of MCQs.
Check our prior analysis on step 3 score reports http://www.ccsworkshop.com/About_us.html . This has not changed much on new Step 3. Please check the back portion of the score report of one of the students who failed with 184. Note how this student has failed despite doing fairly well on MCQs including the dreaded Bio-statistics ( Image below) It goes on to prove CCS can not be neglected and you must score at least above average to do very well on step 3.
High-yield Archer Question bank and lectures now available at https://ArcherReview.com

Filed under: Uncategorized | Tagged: NEW USMLE STEP 3 CHANGES, USMLE STEP 3 NEW, USMLE STEP 3 NEW PASSING SCORE | 1 Comment »
Posted on March 26, 2015 by Archer USMLE Reviews
468) An 18-month-old girl is brought to the hospital by her parents because of a high fever and generalized “body shaking” 2 hours ago. The parents say that she was “pretty fussy” all day and her temperature at that time was 37.3 °C (99.2 °F). Over the past few hours, she began to “burn up,” with her temperature spiking to 39.7 °C (103.4 °F). They put her into her crib to go to sleep, and they heard “banging” coming from the baby monitor in their bedroom. When they arrived in her room 10 seconds later, her entire body was “shaking”. It lasted about 2 minutes, and did not recur. She was lethargic and drowsy for 5 minutes after the “shaking”. Nobody in the family has ever had a seizure before. Her temperature is 39.3 °C (102.8 F). Neurologic examination is unremarkable. A chest x-ray shows a left lower lobe consolidation.
A lumbar puncture shows:
Color clear
Glucose 55 mg/ml
Protein 22 mg/ml
Lymphocytes 4/ml
The parents are concerned about the severity of their daughter’s condition and what they can expect in the future. The most appropriate response is which of the following?
| A. “Luckily, you got to the hospital in time to avoid complications such as hydrocephalus, hearing loss, speech or developmental delays, and mental retardation.” |
| B. “Since you do not have a family history of febrile seizures, chances are that she will go on to have idiopathic epilepsy.” |
| C. “This episode is due to her pulmonary infection, and it will never recur.” |
| D. “Your daughter will most likely have a complete recovery and there is only a very small chance that she will develop epilepsy.” |
| E. “Your daughter’s condition is very serious and a full evaluation for epilepsy is indicated at this time.” |
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer pediatrics, USMLE STEP3 PEDIATRICS | 7 Comments »
Posted on March 26, 2015 by Archer USMLE Reviews
| A 32-year-old man comes in regularly because his back hurts. You suspect malingering because he continually requests narcotic pain relievers and imaging studies of his spine. He has come 5 times in the last 6 months with the same problem, typically stating that the pain improves with exercise and leaning forward but is worse at night; pain also occurs at times in his buttocks and does not improve with rest. On physical examination you find no abnormalities of the spine besides inflexibility of the lower spine when he leans forward. Neurological examination is normal, including the absence of pain on a straight-leg raise test. X-ray of the spine and sacroiliac joint is normal. All blood tests including rheumatoid factor, ESR, and C-reactive protein are normal. Today, he again claims to have only minimal improvement with ibuprofen. He is requesting narcotics and a “doctor’s note to get out of work.” What is the next best step in management? |
| A. MRI of the sacroiliac joint |
|
| B. Anti-cyclic citrullinated peptide (anti-CCP) |
|
| C. HLA-B27 testing |
|
| D. Methotrexate |
|
| E. Report to employer for malingering |
|
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Rheumatology, USMLE step 3 Rheumatology | 9 Comments »
Posted on March 23, 2015 by Archer USMLE Reviews
466. A 60-year-old woman presents to the office with hoarseness, shortness of breath, cough, and bilateral ear pain for the past 3 days. She has experienced similar, but less severe symptoms in the past week. Her past medical history is significant for moderate arthritis of the knees ankles, and wrists for the past 20 years Vital signs are:
temperature 37 C (98.6 F)
blood pressure 120/90 mm Hg
pulse 82/min
Respirations 12 breaths/min
On physical examination, the external ears are tender to touch with the exception of the lobule of the ear. There is no hearing loss. There is a saddle-nose deformity. The lungs have minimal bibasilar rhonchi. Abdominal examination reveals a normal-size spleen and liver. The ankle and knee joints are tender but not erythematous. Chest x-ray demonstrates focal tracheal narrowing. Which of the following is the most appropriate management for this patient at this time?
A An arthrocentesis of the knee joint
B Order knee and ankle x-rays
C Prescribe corticosteroids
D Prescribe nonsteroidal anti-inflammatory drugs
E Schedule a bronchoscopy
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Rheumatology, USMLE step 3 Rheumatology | 19 Comments »
Posted on March 23, 2015 by Archer USMLE Reviews
465) A 6-year-old boy is brought to the emergency department 2 hours after he was bitten by a peer in kindergarten in a fight. The child’s mother is frantic and wants to “sue the center for allowing such behavior!” The child is up-to-date on his vaccinations. The other child is very healthy and his vaccinations are current. On physical examination, there is puncture wound on the right hand. You gently clean the wound and consult with the hand surgeon who says that there does not appear to be any damage to the nerves, muscles, tendons, or joints of the hand. What is the most appropriate next step?
A. close the wound with a suture
B. obtain a wound culture
C. provide ampicillin-sulbactam
D. send him home with a follow-up appointment within 48 hours
E. vigorously irrigate and debride the wound
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Infectious Diseases, internal medicine board review, USMLE STEP 3 INFECTIOUS DISEASES | 13 Comments »
Posted on March 21, 2015 by Archer USMLE Reviews
464) A 68 year old man with history of Diabetes Mellitus type II and diabetic gastroparesis is evaluated in your office for lack of appetite, nausea, vomiting, weight loss and a feeling full after eating small amounts of food material. He has lost 10 lbs weight in past 2 months. He denies any rectal bleeding or melena. He does report some epigastric discomfort. On examination, he appears in no distress, blood pressure is at 120/80. Abdomen is mildly distended with slight tenderness in upper abdomen. Patient is admitted and started on IV hydration. A plain X-ray abdomen is shown below. Gastroenterology is consulted and an upper endoscopy is pending. What is the most appropriate evidence-based next step?

A) Arrange for Laparoscopy
B) Endoscopic removal
C) Give Coca-Cola
D) Give Pepsi or any other soda
E) Metoclopramide
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER GASTROENTEROLOGY, USMLE STEP 3 GASTROENEROLOGY | 21 Comments »
Posted on March 14, 2015 by Archer USMLE Reviews
463) A 48 year old woman presents to the emergency room with significant pain in the epi-gastrium and right upper quadrant. The pain has been persistent for the past 8 hours. She rates her pain at 8/10 in intensity. She reports some nausea but has not vomited. She has no jaundice. She denies any cough or burning urination or hematuria. On examination, she is obese, temperature is 99.9 F, blood pressure is at 120/80. She does not have any pallor or icterus. She lies still on the examination table since any movement aggravates her pain. She demands pain medication before you can examine her. An ultrasound of the abdomen is pending. What is the most appropriate next step?
A) Tell her that physical exam is done before analgesics since analgesia may obscure the diagnosis.
B) Give her morphine sulfate
C) Give her Meperidine
D) Obtain Urine drug screen
E) Tell her that you will not treat her pain without doing physical exam first
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer surgery, usmle step 3 surgery | 11 Comments »
Posted on March 4, 2015 by Archer USMLE Reviews
462) A 39 year old man is seen in your office for swelling of the abdomen and shortness of breath of several months’ duration. He is an active smoker and smoked about 1 pack per day for 20 years. He drinks alcohol occasionally. He denies any cough or fever. On examination, he is afebrile with a temperature of 98.1F , respiratory rate of 18/min, pulse 88/min and blood pressure at 120/70 mm Hg. Cardiac examination reveals regular heart sounds and no S3 gallop. Chest examination shows decreased breath sounds, increased antero-posterior diameter and hyper-resonance to percussion. Abdominal exam is significant for fluid wave and shifting dullness. Liver is enlarged. No splenomegaly.
Labs reveal :
Hemoglobin : 14.2gm%
Serum creatinine 1.0mg%
Albumin 3.2gm%
Total bilirubin 1.0 mg%
Alkaline phosphatase 90 U/L
AST ( Aspartate aminotransferase) 160 U/L
ALT (Alanine aminotransferase) 190 U/L
Which of the following is most helpful in establishing the diagnosis
A. Serum Ferritin
B. Serum Ceruloplasmin leve
C. Anti-Actin and Smooth muscle antibodies
D. 2D Echocardiogram
E. Serum Alpha-1 anti-trypsin level.
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER GASTROENTEROLOGY, USMLE STEP 3 GASTROENEROLOGY | 9 Comments »
Posted on March 4, 2015 by Archer USMLE Reviews
Q461) A clinical trial is evaluating an investigational new drug (Drug A) as compared with standard therapy (Drug B) in patients presenting to the emergency department with myocardial infarction (MI). The study enrolled a total of 3,000 subjects, 1,500 in each study arm. Follow-up was conducted at 45 days post-MI. The following are the results of the trial:
| Endpoints |
Drug A |
Drug B |
P-Value |
| Primary:
· Death from cardiovascular causes |
134 |
210 |
0.03 |
| Secondary:
· Hyperkalemia |
57 |
70 |
0.4 |
What is the relative risk of death from cardiovascular cause? (Round to the nearest whole number)
A. 36%
B. 42%
C. 57%
D. 64%
E. 72%
Filed under: Uncategorized | Tagged: Archer Bio-statistics, USMLE Step 3 Bio-statistics | 11 Comments »
Posted on February 23, 2015 by Archer USMLE Reviews
460)
| A 65-year-old woman with a previous medical history of diabetes and rheumatoid arthritis underdoes an exploratory laparotomy for perforated sigmoid diverticulitis. Her postoperative stay is prolonged because of pneumonia, but is extubated on the fifth postoperative day and the next day discharged from the ICU to the floor. You are called by the nurse to check her wound as she noticed a new serous discharge. Her temperature is 37.0 C (98.6 F), pulse 100 beats/min, blood pressure 140/80 mm Hg, and blood glucose by finger stick 240 mg/dl. Abdomen is soft and non-tender. There is no induration, erythema, or crepitus of the wound. Copious amounts of serous brownish liquid are expressed from the wound on gentle pressure. Fluid is expressed spontaneously on coughing from both upper and lower parts of the wound. Which of the following is the most appropriate next step? |
| A. Start the patient on an insulin drip |
|
| B. Stop anticoagulation with subcutaneous heparin |
|
| C. Order dry dressings to be continued |
|
| D. Prescribe a 1-week course of antibiotics |
|
| E. Schedule the patient for wound exploration in the operating room |
|
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer surgery, usmle step 3 surgery | 14 Comments »
Posted on February 23, 2015 by Archer USMLE Reviews
| 459) A 56-year-old man comes to the clinic complaining of postprandial nausea, occasional vomiting, and abdominal pain. Upon further questioning, he describes intermittent diarrhea and cramps, as well as diaphoresis, palpitations, and flushing. Three months ago he underwent resection of the distal stomach and proximal duodenum and gastrointestinal reconstruction due to complicated peptic ulcer disease. Prior to the operation, he had similar abdominal pain but it was usually relieved, not worsened with eating. Vital signs and physical examination are unremarkable. The most appropriate next step in the management of this patient is which of the following? |
| A. Begin a trial of somatostatin |
|
| B. Counsel the patient about his diet |
|
| C. Determine the patient’s gastrin level |
|
| D. Order a CT scan of the abdomen |
|
| E. Schedule a repeat endoscopy |
|
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer surgery, usmle step 3 surgery | 5 Comments »
Posted on February 23, 2015 by Archer USMLE Reviews
| 458) A 65-year-old man is brought to the emergency department after having two large bowel movements over the past hour that consisted of bright red blood and no stool in the past 3 hours. He denies any abdominal pain or nausea, but does recall having crampy abdominal discomfort after eating over the last several days. His past medical history is significant for hypertension and diabetes and his medications include lisinopril, metoprolol and metformin. He had a colonoscopy at the age of 55 which was unremarkable. On presentation, his temperature is 37.0°C (98.6°F), blood pressure is 80/60 mm Hg, and pulse is 110/min. His abdomen is soft and nontender. There is no guarding or rebound tenderness. There is fresh red blood in the rectum, but there are no palpable masses. Intravenous fluid boluses are started. Which of the following is the most appropriate next step in the management of this patient? |
| A. Perform upper gastrointestinal endoscopy |
|
| B. Order a CT scan of the abdomen |
|
| C. Order a nuclear bleeding scan of the colon |
|
| D. Perform a colonoscopy |
|
| E. Perform nasogastric aspiration |
|
|
|
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER GASTROENTEROLOGY, archer surgery, USMLE STEP 3 GASTROENEROLOGY, usmle step 3 surgery | 14 Comments »
Posted on January 24, 2015 by Archer USMLE Reviews
A 33 year old gravida 2 para 1 female presents for prenatal care at 35 weeks gestation. She was previously seen at 8 weeks gestation for her only prenatal visit. She has had weight gain of only 21 pounds during her pregnancy, but reports her appetite has been good. She has experienced heat intolerance and fatigue for several months. Her heart rate is 112, blood pressure is 124/86 mm Hg. On examination, she has fullness in her neck and exhibits a fine tremor. You should advise this patient that her child will be at risk for which of the following complications ?
a. Thrombocytopenia
b. Macrocephaly
c. Heart failure
d. Heart block
e. Constipation
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: archer obstetrics, usmle step 3 gynecology and obstetrics, usmle step 3 obstetrics | 5 Comments »
Posted on January 19, 2015 by Archer USMLE Reviews
Your patient is a 48-year-old postmenopausal female with recent onset of stage I hypertension. She has a history of osteoporosis. On exam, her blood pressure is 155/90, heart rate is 80 and regular, and respirations are 16. Cardiovascular examination is unremarkable. She has no other medical history. She has previously been controlling her hypertension with lifestyle modifications and diet. Which of the following medications is the best choice for initial pharmacological treatment of her hypertension?
A. Calcium channel blocker
B. Thiazide diuretics
C. Angiotensin receptor blocker
D. Alpha-adrenergic blocker
E. Angiotensin converting enzyme inhibitor
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer cardiology, archer general internal medicine, internal medicine board review, USMLE STEP 3 CARDIOLOGY | 4 Comments »
Posted on January 17, 2015 by Archer USMLE Reviews
You are evaluating a 63-year-old female with a complaint of abdominal pain radiating to her back. She has diminished appetite and pruritus. Her past history is significant for a 40-year history of tobacco use. She has three glasses of wine once a week.
The patient is afebrile, with blood pressure of 123/85, and her heart rate is 74. She appears jaundiced, with scleral icterus. Her abdomen is tender to palpation in the epigastric region, and her liver is palpable 2 cm below the right costal margin. Laboratory tests reveal total bilirubin of 6.0. direct bilirubin of 4.8, alkaline phosphatase of 1000, and AST is 42, with and ALT of 40. Which of the following initial diagnostic test will you order?
A. Abdominal ultrasound
B. Percutaneous liver biopsy
c. Percutaneous transhepatic cholangiography
D. CT abdomen and pelvis
E. Endoscopic retrograde cholangio-pancreatography.
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: ARCHER GASTROENTEROLOGY, internal medicine board review, USMLE STEP 3 GASTROENEROLOGY | 8 Comments »
Posted on January 11, 2015 by Archer USMLE Reviews
Your patient presents at 33 weeks gestation. She is a 27 year old gravida 2 para 1 female with a previous pregnancy complicated by pre-eclampsia, with a vaginal delivery of a healthy infant at 37 weeks. Her current pregnancy has been uncomplicated, but today she is experiencing abdominal pain and uterine contractions that began 5 hours prior to presentation. She states that she has had some fluid leaking from her vagina since the previous evening, but denies vaginal bleeding, trauma, urinary urgency or frequency.
Her temperature is 101.6 degrees Fahrenheit. Blood pressure is 98/60, heart rate is 100/minute, with respirations of 18/minutes. On pelvic examination, she has clear fluid in the posterior fornix, which causes nitrazine paper to turn blue in color. She is dilated to 4 centimeters and her cervix is 60% effaced. Her uterus is diffusely tender on examination and the fetal heart rate is 175 beats per minute, with frequent accelerations. Maternal white blood cell count is 13,000/mm3.
What is the next step in the management of this patient?
A. Initiation of ciprofloxacin
B. Urine culture
C. Initiation of ampicillin and gentamycin
D. Cesarean delivery
E. Induction of labor.
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Gynecology, archer obstetrics, obgyn, usmle step 3 gynecology and obstetrics, usmle step 3 obstetrics | 3 Comments »
Posted on January 9, 2015 by Archer USMLE Reviews
Your patient is a gravida 1 para 0, 19 year old female at 33 weeks gestation that presents with a complaint of heavy vaginal bleeding and severe abdominal pain for an hour. Her pregnancy has been uneventful, and she is in good health, although she continues to smoke cigarettes, admitting to one pack per day.
Blood pressure is 110/80 with a heart rate of 110. The patient is afebrile, with respirations of 20/minute. Examination reveals a contracted uterus with a closed cervical os. The patient expels a large amount of blood from her closed cervix during the examination. Fetal heart rate monitoring reveals severe late decelerations. What is the best treatment option for this patient?
A. Expectant management with bed resT
B. Terbutaline
C. Oxytocin
D. Magnesium sulfate
E. Caesarean section delivery
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Gynecology, archer obstetrics, archer ostetrics and gynecology, obstetrics, usmle step 3 gynecology and obstetrics | 8 Comments »
Posted on January 9, 2015 by Archer USMLE Reviews
Your patient is a gravida 2 para 2 female with a complaint of a vaginal discharge, vulvar irritation, and dysuria. Her symptoms have recurred 4 times within the past year. She had a recent urinary tract infection that was treated with ciprofloxacin, but is in otherwise good health. She is afebrile with normal vital signs, and pelvic examination reveals vulvar and vaginal erythema with a thick white discharge in the vaginal vault. KOH prep reveals pseudo hyphae. Which of the following treatments is most appropriate for this patient?

a. Oral fluconazole 150 mg x 1
b. Probiotic lactobacilli
c. No treatment is necessary
d. Oral Fluconozole induction followed by maintenance.
e. Topical miconazole x 7 days
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer Gynecology, archer ostetrics and gynecology, usmle step 3 gynecology | 11 Comments »
Posted on October 19, 2014 by Archer USMLE Reviews
450) A 74 year old man with poorly controlled Diabetes Type II presents to your office with complaints of severe right ear pain since yesterday night. The Pain was particularly worse in the night and interfered with his sleep. He took some Advil but pain persisted. He also reports headache on right temporal aspect. On examination, his right external auditory canal is slightly red and swollen and extremely tender to touch. There is some white debris and granulation tissue at the junction of bone and the cartilage. Laboratory studies show normal WBC count with no left shift. Which of the following additional tests is useful in supporting the diagnosis?
A) Comprehensive metabolic panel
B) Erythrocyte Sedimentation Rate
C) Anti-nuclear antibodies
D) Hemoglobin A1C.
E) Screening nares for MRSA ( Methicillin Resistant Staph. Aureus)
451) What is the most important next step in management?
A) Oral Cephalexin
B) Surgical resection
C) Intravenous Ciprofloxacin
D) Intravenous Ceftriaxone
E) Ciprofloxacin ear drops
Filed under: USMLE STEP III QUESTION BANK, USMLE Test Prep | Tagged: Archer ENT, Archer Infectious Diseases | 10 Comments »
Posted on September 22, 2014 by Archer USMLE Reviews
Archer USMLE Theory Courses have placed huge emphasis on Preventive Medicine, Ethics, Patho-physiology which are going to be tested even more frequently on restructured USMLE Step 3.
Please note USMLE Step 3 CCS will be administered on Day 2 and content , software and format of CCS will remain the same as before!! You may access CCS Workshop at CCS Workshop
Changes to USMLE 2014 – 2015
As medicine and medical education have changed over the years, so have USMLE examinations evolved since they were first administered in 1992. This is a brief summary of planned changes for the next few years.
USMLE STEP 3
What WILL change?
Beginning November 3, 2014, examinees will:
Be able to take the exam on two consecutive or non-consecutive days;
NOT need to apply for Step 3 under the eligibility requirements of a specific medical licensing authority;
See increased numbers of items that assess an expanded range of competency-based content, including foundational science essential for effective healthcare; biostatistics, epidemiology, and population health; literature interpretation; medical ethics; and patient safety. The two exam days will be named Step 3 Foundations of Independent Practice (FIP) and Step 3 Advanced Clinical Medicine (ACM).
What WILL NOT change?
The Step 3 exam will continue to:
Focus on knowledge and application of the biomedical and clinical sciences necessary for independent patient care;
Include multiple-choice questions and computer-based case simulations;
Be administered over two days, for a total time comparable to current testing time;
Result in a single score (with graphical performance profile information) and a single pass/fail outcome after completion of both examination days.
Be administered at Prometric test centers throughout the United States.
Important to Note : ( Taken from USMLE Official website) :
Applications for the restructured Step 3 examination will be accepted starting on August 4, 2014.
No Step 3 examinations will be administered during most or all of October 2014.
Administration of the restructured Step 3 exam will begin on November 3, 2014.
There will be a score delay following introduction of the restructured Step 3 examination on November 3, 2014. The duration of the score delay will be determined by examinee volume during the early months of exam administration. Based on historic trends, we estimate that scores for Step 3 exams taken on or after November 3, 2014 will be released in April 2015.
GOOD LUCK WITH YOUR EXAM!
Filed under: Uncategorized | 2 Comments »
Posted on February 8, 2014 by Archer USMLE Reviews
Q448 ) A 78 year old man presents to emergency room with severe pain in his right lower extremity. Pain began after he stumbled and fell on a sidewalk. He does not report pain anywhere else and did not lose consciousness. No tingling or numbness in either extremity. He reports inability to bear weight because it is extremely painful. His past medical history is significant for coronary artery disease and hypertension. He does report about 8lbs weight-loss in the past 1 month. On examination, he is afebrile. There is tenderness in the right thigh area. Laboratory investigations reveal Hemoglobin 10.5gm%, Platelet count 110k/ul, Calcium at 10.4 mg/dl ( N = 9.0 to 10.5 mg/dl) , Serum creatinine 2.0 mg/dl, Total protein 4.5 gm/dl, Albumin 2.0gm/dl and Ferritin 200 ng/ml. Liver function tests including Alkaline phosphatase are within normal limits. Whole body bone scan is negative for any lesions. Serum protein electrophoresis is normal with out any monoclonal spike.
An x-ray of the femur is shown below :

448) Which of the following is most helpful in diagnosing this condition?
A) Colonoscopy
B) DEXA scan
C) 24 hour urine electrophoresis
D) Vitamin D level
E) Parathyroid hormone level
449) Which of the following is likely to explain the patient’s findings?
A) Metastatic colon cancer
B) Multiple Myeloma
C) Osteoporosis
D) Secondary Hyperparathyroidism
E) Metastatic Prostate cancer
Filed under: Uncategorized, USMLE Test Prep | Tagged: Archer endocrinology, archer general internal medicine, Archer oncology, USMLE STEP 3 ONCOLOGY | 26 Comments »















